Barium Bromide
Barium bromide is a chemical compound and it dissolves in water and toxic. It looks like a deliquescent orthorhombic crystals. It can cause severe poisoning on ingesting and some other water soluble barium salts are considered as the toxic. It reacts with the oxygen, sulfur and carbon to form barium compounds. It should be the pure form of metallic alkaline in nature. The systematic IUPAC name is known as Barium bromide. The chemical or molecular formula of Barium bromide is BaBr2.
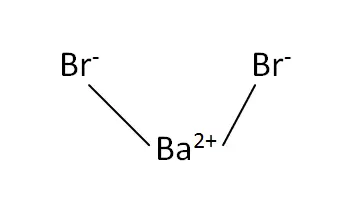
Structural Formula
This is the structural formula of the Barium bromide:
Chemical Formula
The chemical formula of the Barium bromide is BaBr2.
Preparation Method
When the barium sulfide is reacted with the hydrobromic acid it gives the result of formation of barium bromide. It gives the hydrogen sulfide as the byproduct. In another method the barium carbonate is reacted with the hydrobromic acid it gives the formation of barium bromide. Carbon dioxide and water is the byproduct.
BaS + 2 HBr → BaBr2 + H2S
BaCO3 + 2 HBr → BaBr2 + CO2 + H2O
Physical Properties
| Melting point | 857C |
| Boiling point | 1835C |
| Molecular weight | 297.14g/mol |
| Density | 4.78g/cm3 |
| Solubility in water | 92.2g/100mL(0C) |
| Crystal structure | Orthorhombic |
| Magnetic susceptibility | -92.1×10-6cm3/mol |
| Appearance | White solid |
Chemical properties
Barium bromide has contains the three bonded covalently. It is white crystalline powder. The hydrogen bond donors is equivalent to zero and the hydrogen bond acceptors is equivalent to 2. The boiling boint is very high and the melting point is low. It is orthorhombic in structure. It is soluble in water. it has a medium molecular weight and high density.
Uses
Barium bromide is used precursor in chemicals. It is also used in photography. It is widely used in manufacturing of other chemicals. It is used in the process of fractional crystallization. It is used in production of phosphorous.