Ammonium Sulfide Formula
Ammonium sulfide is a solution that is used for to store the liquid securely in a small bottle like container which is sealed in a class called ampoule. It is an inorganic compound in nature. It is more corrosive and hazardous to the environment. The other names that has been referred as rarely called diammonium sulfide. The systematic IUPAC name is known as ammonium sulfide. The chemical or molecular formula of ammonium sulfide is (NH4)2S. this is also known as the ‘stink bomb’.
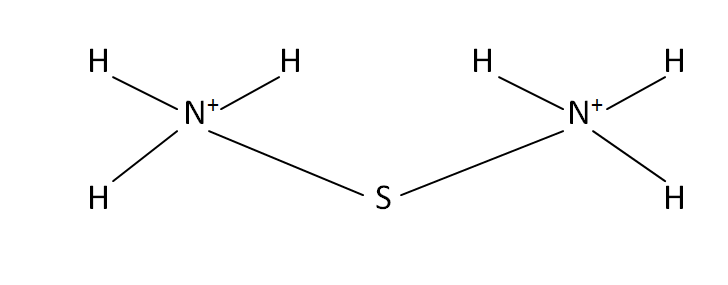
Structural Formula
This is the structural formula of the ammonium sulfide:
Chemical Formula
The chemical formula of the ammonium sulfide is (NH4)2S.
Structure
It is the formation of with the help of 1 centered sulfur atom that contains around ammonium cations NH4+ attached to the sulfur.
Preparation Method
When the ammonium sulfide is reacted of ammonium hydroxide with the excess of hydrogen sulfide that can be formed the ammonium hydrosulfide. This is called as an intermediate product. Then further it can be treated with the same quantity of ammonia with the hydrosulfide that gives the ammonium sulfide.
HS + exc NH3 → (NH4)2S
Physical Properties
| Molecular weight | 68.154g/mol |
| Density | 0.997g/cm3 |
| Melting point | Decomposes at ambient temperatures |
| Solubility in water | 128.1g/100mL |
| Appearance | Yellow crystals hygroscopic |
| Solubility | Solubility in alcohol |
Chemical Properties
It has highly toxic and highly inflammable. It gives an pungent and unpleasant smell. When the ammonium sulfide is dissociation with the reaction of ammonia and hydrogen sulfide that as follows.
(NH4)2S → HS+NH3
Uses
It is used for the manufacturing in the developing photographs . the ammonium sulfide is used in the textile industry. When it is used for making the metals that is added to the bronze for getting stronger. It is toxic and keep out reach of the children.