Ammonium Iodide Formula
Ammonium iodide is the chemical compound which it crystallizes in cubes and it is easily soluble in water. With the use of moisture air decomposes and turns the yellow colour with the liberation of iodine. It is also soluble in ethanol. When the prepared action of hydroiodic acid on ammonia. The systematic IUPAC name is known as ammonium iodide. The chemical or molecular formula of ammonium iodide is NH4l.
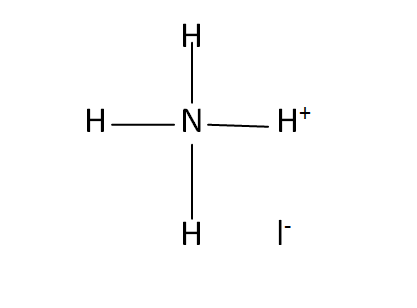
Structural Formula
This is the structural formula of the ammonium iodide:
Chemical Formula
The chemical formula of the ammonium iodide is NH4I.
Preparation Method
The ammonium hydroxide can be prepared by reacting ammonium with the hydroxide acid or hydrogen iodide gas.
NH4OH+HI → NH4I+H2O
It is decomposed with the ammonium nitrogen triiodide(an explosive).
Physical Properties
| Melting point | 551C |
| Boiling point | 235C |
| Molecular weight | 144.94g/mol |
| Density | 2.51g/cm3 |
| Solubility in water | Soluble |
| Appearance | White crystalline powder |
Chemical Properties
When the crystallization starts by adding ammonia water with the amount of alcohol to obtain a precipitate of potassium sulfate. About 20 percent potassium sulfate combining the filtrates and finally we got the result ammonium iodide. It may sublime into the fire and then cooling on the cold surfaces. The toxic and irritating fumes of the nitrogen may result in the air.
Uses
Ammonium iodide is used to manufacturing for photographic chemicals. It can be used as a penetrating agent and fire suppressant. In medicines fields it is used to preparing the tablets. It is also used in water disinfection agent. It has low toxicity and so does not produce any hazardous activities to the environment.