Ammonium Hydroxide
Ammonium hydroxide is a chemical compound that has the composition of the ammonia cations and hydroxide anions. It is the solution of ammonia in water. This is an alkali substance and in the aqueous of ammonia. It has a significant amount of the ions at the given solution of ammonia hydroxide. Constantly it balanced the mixturing of the solutions.The systematic IUPAC name is known as ammonium hydroxide. The chemical or molecular formula of ammonium hydroxide is NH4OH.
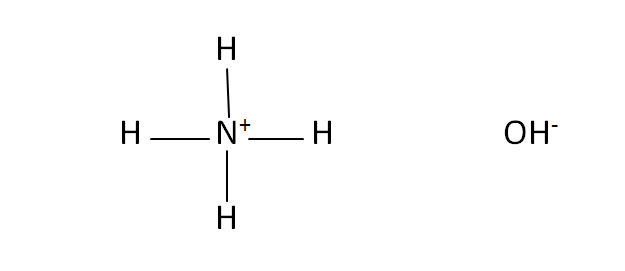
Structural Formula
This is the structural formula of the ammonium hydroxide:
Chemical Formula
The chemical formula of the ammonium hydroxide is NH4OH.
Preparation Method
When the ammonia is reacted with the water that gives the result of the ammonium hydroxide. In aqueous solution the equilibrium of this solution is converted to the more number of ph level. It is basically consists of the base ionization constant.
NH3 + H20 → NH4+ + OH–
Physical Properties
| Melting point | 215K |
| Boiling point | 310.8K |
| Molecular weight | 35.04g/mol |
| Density | 0.91g/cm3 |
| Solubility in water | miscible |
| Appearance | Colourless liquid |
Chemical Properties
The melting and boiling point is very low. It is hexagonal in structure. It is soluble in water. It has a low density and low molecular weight. It is colourless liquid in the appearance. It attains the saturation point at the concentration of dissolved ammonia.
Uses
Ammonium hydroxide is used to manufacturing of chemical fertilizers. It is also used as an organic and inorganic chemicals containing nitrogen. It is widely as production of chloroamine. It is a cleaning agent. It is usually used as a manufacturing a furniture darkening.