Ammonium Dichromate
Ammonium dichromate is an inorganic compound and has a red orange crystallization. It could leaves the green residue at the room temperature. Ammonium dichromate is more sensitive to light and acts as a reliable oxidizing agent. These have consists of ammonium ions and dichromate ions. Due to the decomposition of the material it would be ruptured. The systematic IUPAC name is known as ammonium dichromate. The chemical or molecular formula of ammonium dichromate is (NH4)2Cr2O7.
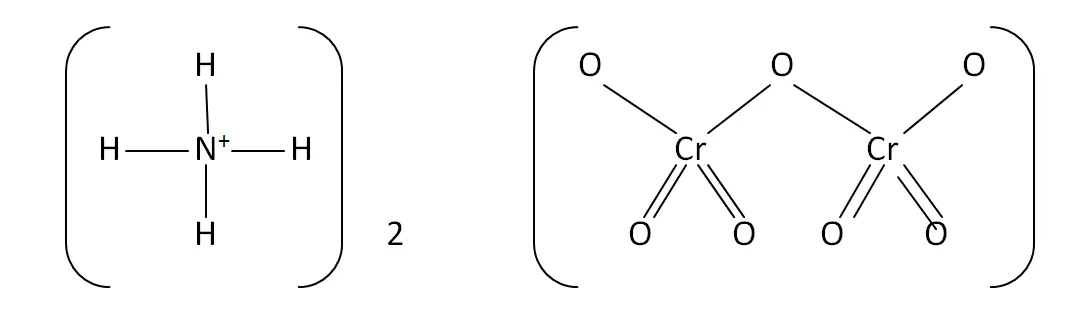
Structural Formula
This is the structural formula of the ammonium dichromate:
Chemical Formula
The chemical formula of the ammonium dichromate is (NH4)2Cr2O7.
Preparation method
When the ammonium dichromate is undergoes to the decomposition at room temperatures it give the result of chromium III oxide and nitrogen gas formation. It is more hazardous to the environment. It has contains a single type of ammonium salts at the same symmetry to the typical of hydrogen bonds. It is crystalled in nature. These hydrogen bonds is symmetrical in naturally point of ammonium salts.
(NH4)2Cr2O7 → Cr2O3 + N2 + 4H2
Physical Properties
| Melting point | 180C |
| Boiling point | decomposes |
| Molecular weight | 252.07g/mol |
| Density | 2.115g/cm3 |
| Solubility in water | 18.2g/100mL |
| Solubility | Insoluble in acetone
Soluble in alcohol |
| Crystal structure | Monoclinic |
| Appearance | Orange red crystals |
Chemical Properties
The melting point is very low and the boiling point is decomposed. It is monoclinic in structure. It is insoluble in acetones and alcohols. It has a low density and medium molecular weight. It is orange red crystals in the appearance.
Uses
Ammonium dichromate is mainly used in lithography. It is a sensitizing solutions. It is used as a pyrotechnics, lithograpy, photoengraving. It is widely used magnetic recording material. It is used in agricultural field approved as a pesticide. Usually used as a mordant Dyeing and pigment.