Ammonium Carbonate
Ammonium carbonate is a chemical compound that has consists of ammonia and carbonate ions. It is a colourless crystalline solid and has a sharp ammonium smell agent. It is acidic in nature and acts as basic. It is soluble in water and non combustible substances.The systematic IUPAC name is known as ammonium carbonate. The chemical or molecular formula of ammonium carbonate is (NH4)2CO3. It is also known as bakers ammonia.
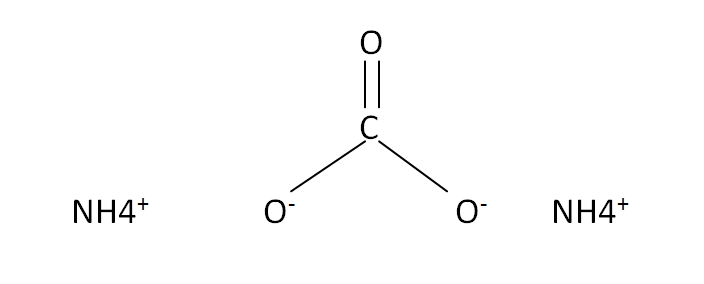
Structural Formula
This is the structural formula of the ammonium carbonate:
Chemical Formula
The chemical formula of the ammonium carbonate is (NH4)2CO3.
Preparation Method
When the ammonia is reacts with the carbon dioxide in the aqueous form it gives the results of ammonium carbonate. Further it can be decomposed to the ammonium bicarbonate and ammonia. It also crystallizes in an ammonium solution to exposed in the carbon dioxide rich atmosphere.
(NH4)2CO3 → NH4HCO3 + NH3
NH4HCO3 → H2O + CO2 + NH3
Physical Properties
| Melting point | 58C |
| Boiling point | decomposes |
| Molecular weight | 96.09g/mol |
| Density | 1.50g/cm3 |
| Solubility in water | Soluble |
| Crystal structure | hexagonal |
| Magnetic susceptibility | -42.50×10-6cm3/mol |
| Appearance | White powder |
Chemical Properties
The melting and boiling point is low and decomposed respectively. The magnetic susceptibility is very low. It is hexagonal in structure. It is insoluble in alcohols. It has a low density and low molecular weight. It is white powder in the appearance.
Uses
Ammonium carbonate is widely as a leavening agent. It is used to degrading to gaseous ammonia and carbon dioxide when it is heated. It is non combustible and soluble in water. It is very harmful to environment. It is used as the main component of the smelling salts.