Ammonium Bicarbonate
Ammonium bicarbonate is an inorganic compound that has white crystalline solid in nature. It has consists of ammonia as cations and bicarbonate as anions. It has a strong smell of ammonia and which is mildly basic compound. Ammonium bicarbonate is soluble in water and insoluble in various organic solvents. The systematic IUPAC name is known as ammonium hydrogen carbonate. The chemical or molecular formula of ammonium bicarbonate is NH4HCO3 . It is also known as powdered baking ammonia.
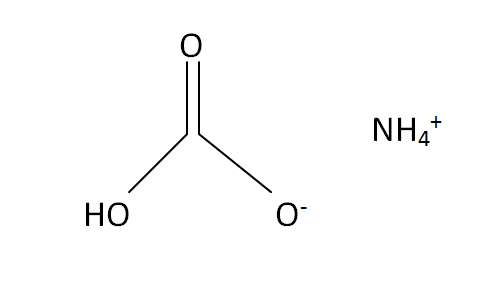
Structural Formula
This is the structural formula of the ammonium bicarbonate:
Chemical Formula
The chemical formula of the ammonium bicarbonate is NH4HCO3.
Preparation Method
The ammonium bicarbonate is prepared when it is reacting with carbon dioxide and water. it allows the precipitation of the product when it is ready to dissolve in water on the aqueous form. It is an exposure to the thermally unstable. In an endothermic process, it has decomposed at 36C with the ammonia, carbon dioxide and water. it is insoluble in acetones and alcohols.
CO2 + NH3 + H2O → (NH4)HCO3
Physical Properties
| Melting point | 41.9C |
| Boiling point | decomposes |
| Molecular weight | 79.056g/mol |
| Density | 1.586g/cm3 |
| Solubility in water | 11.9g/100mL(0C) |
| Solubility | Insoluble in methanol |
| Appearance | White crystalline solid |
Chemical Properties
It has exhausted more chemicals to hazardous for the environment. It is very difficult to breathing, coughing, sneezing and irritating to the eyes. It has been decomposed at the boiling point. The melting point is very low. It is insoluble in methanol. In its nature it is white crystalline solid compound.
Uses
Ammonium bicarbonate is mostly used in the food process. It is antacid.it is used in cough syrup ,baking soda. It is also used in agricultural field like fertilizers. Ammonium bicarbonate is used in manufacturing of dyes,ceramics, plastics. It is mainly used in pharmaceutical production and also a fire retardants.