Ammonium Acetate
Ammonium acetate is a white crystalline solid compound and it is a slightly odourless. It is obtained by the reaction of ammonia with acetic acid. It is mainly used for chemical analysis, food preservatives and pharmaceutical industry. The systematic IUPAC name is known as ammonium acetate. The chemical or molecular formula of ammonium acetate is NH4CH3CO2. It is also known as the spirit of mindererus.
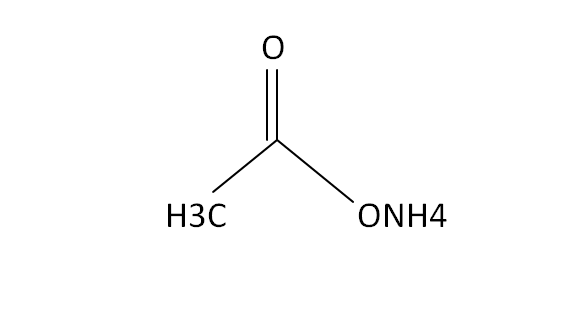
Structural Formula
This is the structural formula of the ammonium acetate. It is the combination of weak acid and weak base to form a buffer solution. At low pressures this chemical components is volatile because it is used to replace the new buffer solution with non-volatile compounds.
Chemical Formula
The chemical formula of the ammonium acetate is NH4CH3CO2.
Preparation Method
There are the two methods for preparing the ammonium acetate that has been as follows. It can be produced by the saturation of glacial acetic acid or CH3COOH with NH3 or ammonia (or) by the neutralization of acetic acid with (NH4)2CO3 or ammonium carbonate. The chemical reaction is as follows.
NH4CH3CO2 → CH3C(O)NH2 + H2O
Physical Properties
| Melting point | 113C |
| Boiling point | 1625C |
| Molecular weight | 77.083g/mol |
| Density | 1.17g/cm3 |
| Solubility in water | 102g/100mL |
| Crystal structure | Orthorhombic |
| Magnetic susceptibility | -41.1×10-6cm3/mol |
| Appearance | White solid crystals, deliquescent |
| Viscosity | 21 |
| Acidity | 9.9 |
| Basicity | 33 |
Chemical Properties
It is more hazardous to the atmosphere or living environment. The viscosity of the ammonium acetate is high and the balance of acidity and basicity has been equal to the share of chemical compounds. The boiling point of the ammonium acetate is very high.
Uses
Ammonium acetate is used for making of explosives. It is used to manufacturing of vinyl plastics. It is a reagent used for analytical chemistry. Ammonium acetate is widely used to preservation of meat. It is mostly used for textile industry for dyeing. It is used to the form of food acidity regulator.