Aluminium Phosphate
Aluminium phosphate is a chemical compound and it occurs as the mineral berlinite. It is used as a catalyst, ion exchangers and molecular sieves. When it is heated to the process of berlinite and it undergoes to the cristoballite forms. This is the framework of the structures of the alumina phosphate that involves to the charged particles. The systematic IUPAC name is known as aluminium phosphate. The chemical or molecular formula of aluminium phosphate is AlPO4.
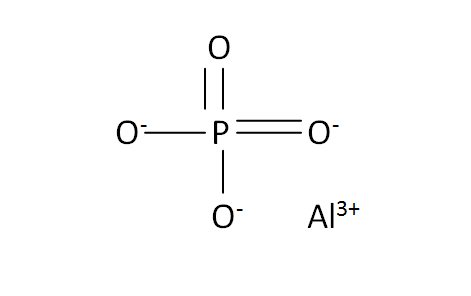
Structural Formula
This is the structural formula of the aluminium phosphate:
Chemical Formula
The chemical formula of the aluminium phosphate is AlPO4.
Preparation Method
When it is reacted with the aluminium with the phosphate that gives the result of the aluminium phosphate. This is one of the main compounds that reacts with the involvement to form in the different sized cavities. In normal use aluminium phosphate and hydroxide salts are acts as the safe antacids. It is used for pigments, corrosion inhibitors, and dental care products.
Physical Properties
| Melting point | 1800C |
| Boiling point | decomposes |
| Molecular weight | 121.9529g/mol |
| Density | 2.566g/cm3 |
| Solubility in water | 1.89×10-9 g/100mL |
| Refractive index | 2.566 |
| Solubility | Very slightly soluble in HCl and HNO3 |
Chemical Properties
The melting point is very high and the boiling point is decomposed. It is very slightly soluble in HCl and HNO3. The refractive index is slightly greater. It has a low density and medium molecular weight. It has more active materials.
Uses
Aluminium phosphate is used in the manufacture of chemicals to enhance the immunogencity. It is also used for water suspension. It is an antacid used for patients loss of phosphate occur in the use of aluminium salts. Aluminium phosphate is used in tablets officially in blood pressure and each tablet weighs in 500mg.