Aluminium Nitrate Formula
Aluminium nitrate is the salt of aluminium which is soluble in water and has formed the white crystalline precipitate. Its appearance is white crystalline colour. This is abundantly found in the earth’s crust and combined to form with together such as silicon, fluorine and oxygen. The systematic IUPAC name is known as aluminium nitrate. The other names are such as nitric aluminium salt, aluminium nitrate and aluminium (III) nitrate. The chemical or molecular formula of aluminium nitrate is Al(NO3)3.
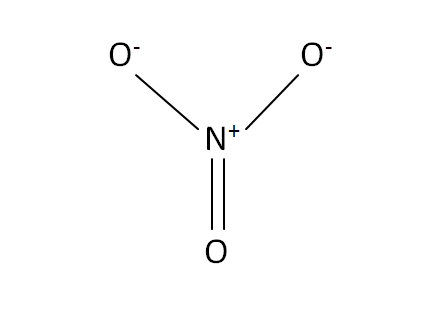
Structural Formula
This is the structural formula of the aluminium nitrate:
Chemical Formula
The chemical formula of the aluminium nitrate is Al(NO3)3.
How to preparation the aluminium nitrate
Aluminium nitrate can be prepared by the metathesis reaction between aluminium sulphate and nitrate salt. When the aluminium sulphate is reacted with the suitable cations such as barium, lead, silver and calcium and it produced the aluminium nitrate.
Al2(SO4)3 + 3Ba(NO3)2 → 2Al(NO3)3 + 3BaSO4
It cannot be synthesized by the concentration of nitric acid.
Physical Properties
| Melting point | 72.8C |
| Boiling point | 135C |
| Molecular weight | 212.996g/mol |
| Density | 1.72g/cm3 |
| Solubility in water | Anhydrous |
| Solubility in ethanol | 8.63g/100ml |
| Refractive index | 1.54 |
| Solubility in methanol | 14.45g/100ml |
| Appearance | White crystal solid |
Chemical Properties
It has odourless chemical compounds. These has contains the hazardous elements and anhydrate products. It is good in taste. It has in pure form the aluminium nitrate. This could be the most abundant in the earth crust. The organic and inorganic chemicals are produced by using this minerals which is reacted with the aluminium nitrate.
Uses
It is used to the manufacturing of powerful oxidizing agent. It is used to extraction of uranium from the other compounds. In petroleum products, this can used to refinining process. It is used as a corrosion inhibitor in the industries. In the transformer it used to laminates the core particle substances. It is used to manufacturing the insulating papers, cathode ray tube heating elements and core laminates.