Aluminium Fluoride
Aluminium fluoride is an inorganic compounds and naturally occurred in the oskarssonite and rosenbergite. At 700C the hydrogen fluoride is treated with the alumina or aluminium oxide to produce the aluminium fluoride. The sublimation process is taken at the certain temperatures at 1272 degrees. So this chemical compound is highly stable. The systematic IUPAC name is known as aluminium fluoride. The chemical or molecular formula of aluminium fluoride is AlF3. The other name is called as aluminium trifluoride.
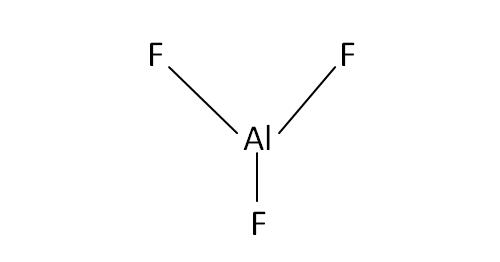
Structural Formula
This is the structural formula of the aluminium fluoride:
Chemical Formula
The chemical formula of the aluminium fluoride is AlF3.
Preparation Method
When the alumina is reacted with the hydrogen fluoride and it produces the aluminium fluoride. In another method it is thermally decomposed the ammonium hexafluroaluminate. This acid is also used to make the aluminium fluoride. It has been also used as a precursor to zeolite. It exists as a trigonal molecules.
H2SiF6 + Al2O3 + 3H2O → 2AlF3 + SiO2 + 4H2O
Physical Properties
| Melting point | 1291C |
| Boiling point | 255C |
| Molecular weight | 83.977g/mol |
| Density | 3.10g/cm3 |
| Solubility in water | Soluble |
| Refractive index | 1.3767 |
| Crystal structure | Rhombohedral |
| Magnetic susceptibility | -13.4×10-6cm3/mol |
| Appearance | White crystalline solid odorless |
Chemical Properties
Aluminium fluoride has a high melting point and low boiling point. It is soluble in water. The refractive index for the aluminium fluoride is balanced. It looks like a white crystalline solid odorless. It has no taste. It contains the rhombohedral structure.
Uses
Aluminium fluoride is used as a catalyst in organic compounds. It is used in low index optical thin film. It is widely used in fermentation process for wine and beer industries. Aluminium fluoride is used in additive for the production of aluminium by electrolysis.