Aluminium Bromide
Aluminium bromide is a chemical compound that has colourless, hygroscopic solid and sublimable substances. The most common form of aluminium bromide is aluminium tribromide. It looks like a white yellowish red compound and has a pungent odour. Aluminium bromide acts as a sol at many organic solvents such as benzene, toluene, nitrobenzene, xylene and hydrocarbons. The systematic IUPAC name is known as aluminium bromide. The chemical or molecular formula of aluminium bromide is AlBr3. It is also known as cupric chloride or aluminium tribromide.
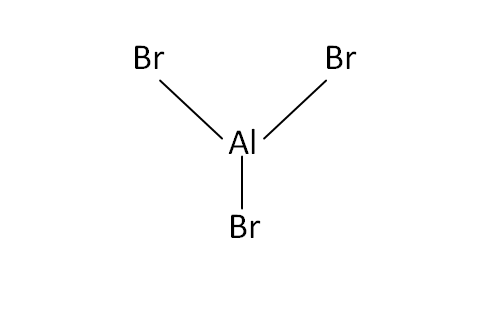
Structural Formula
This is the structural formula of the aluminium bromide:
Chemical Formula
The chemical formula of the aluminium bromide is AlBr3.
Preparation Method
When the hydrogen bromide is reacted with the aluminium that produce the chemical substances known as aluminium bromide. Due to the presence of iron containing impurities it could be easily attain the certain properties. It has contain the smaller size of central atom so does not show the tendency. When it reacts with the water it exposed less vigorously.
2Al + 6 HBr → Al2Br6 + 3 H2
Physical Properties
| Melting point | 97.5C |
| Boiling point | 255C |
| Molecular weight | 266.694g/mol |
| Density | 3.2g/cm3 |
| Solubility in water | Very soluble |
| Odor | pungent |
| Crystal structure | Monoclinic |
| Appearance | White to pale yellow powder |
Chemical Properties
Aluminium bromide is monoclinic in structure. It is commonly heavier group halides which is used to form the large aggregates. Aluminium bromide is reacted quickly with the alcohols and carboxylic acids. When it is heated at 100C it forms the carbon tetrachloride.
Uses
Aluminium bromide is used in medication. It is also to manufacturing the chemicals. Aluminium bromide is used as a catalyst for many chemical process. It is highly reactive materials.