Acetonitrile
Acetonitrile is a chemical compound and it is a colourless liquid. It is used as polar aprotic solvent in organic synthesis and it help for the purification of butadiene. It manufactures the acrylonitrile as the byproduct. It is often called as the methyl cyanide. It is ability to dissolve in the electrolysis process. The systematic IUPAC name is known as Ethanenitrile. The chemical or molecular formula of acetonitrile is C2H3N.
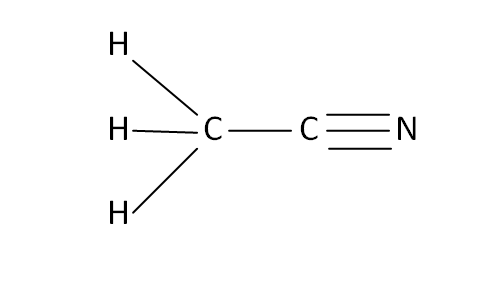
Structural Formula
This is the structural formula of the acetonitrile:
Chemical Formula
The chemical formula of the acetonitrile is C2H3N.
Preparation Method
The acetonitrile is produced from the product of acrylonitrile. It is commonly known as the byproduct and dehydration of the alcohols. It the mixture of carbon monoxide and ammonia with the formation of superacids. When the palladium is heating and it could be lead to the suspension of the substance formation. It rapidly changes the complexes by many other ligands.
PdCl2 + 2 CH3CN → PdCl2(CH3CN)2
Physical Properties
| Melting point | -46 to -44C |
| Boiling point | 81.3 to 82.1C |
| Molecular weight | 41.053g/mol |
| Density | 0.786g/cm3 |
| Solubility in water | Miscible |
| Refractive index | 1.344 |
| Crystal structure | hexagonal |
| Magnetic susceptibility | -28.0×10-6cm3/mol |
| Appearance | Colourless liquid |
| Acidity | 25 |
Chemical properties
The melting and boiling point is low and decomposed respectively. The magnetic susceptibility is very low. It is hexagonal in structure. It is soluble in water as a miscible. It has a low density and high molecular weight. It is colourless liquid in the appearance. It has reached the point of 25 in the level of acidity.
Uses
Acetonitrile is used as a solvent for the extraction of hydrocarbons. It is used in vegetable oil to separate the fatty acid. It is also used in the manufacturing of perfumes and also rubber. It is a solvent used in electro chemical cells. It is widely used in extraction of copper.