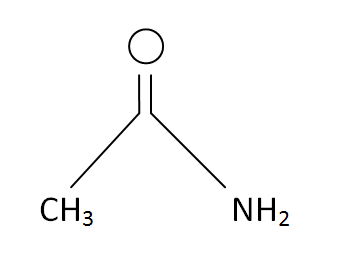
Acetamide Formula
Acetamide is a colourless crystal solid that has no odour taste. In its pure form it has white in colour and the taste is very bitter. It has naturally found in red beetroot. When the acetamide is synthesized with anhydrous acetic acid, dried hydrogen gas in the presence of the reagent is called as the product obtained is acetyl chloride. It act as the intermediate between the acetones. The systematic IUPAC name is known as ethanamide. The chemical or molecular formula of acetamide is C2H5NO.
Structural Formula
This is the structural formula of the acetamide:
Chemical Formula
The chemical formula of the acetamide is CH3CONH2 or C2H5O.
What Method the Acetamide Can be Produced
The acetamide can be produced by the two ways. They are laboratory scale and industrial scale. In the laboratory scale by dehydration process the acetamide can be produced from the ammonium acetate.
[NH4][CH3CO2] → CH3C(O)NH2+H2O
In the industrial scale the acetamide is produced from ammonium acetate or the hydration of the acetonitrile. The acetonitrile which is the byproduct of acrylonitrile.
CH3CH+H20 → CH3C(O)NH2
Physical Properties
| Melting point | 79 to 81C |
| Boiling point | 221.2C |
| Molecular weight | 59.068g/mol |
| Density | 1.159g/cm3 |
| Solubility in water | 2000gL-1 |
| Vapour pressure | 1.3pa |
| Refractive index | 1.4274 |
| Viscosity | 2.052cP(91C) |
| Crystal structure | trigonal |
Chemical Properties
It has a mousy odour and it is in the form of hygroscopic solid. The sharing of electrons to each other from carbonyl, methyl and anime groups to form the acetamide. It is also obtained from the condensation of acetic acid with ammonia. It has bitter in taste.
Uses
Acetamide is used to manufacturing for plasticizer and hygroscopic agent. It can be used as a penetrating agent and fire suppressant. Many organic and inorganic compounds used the acetamide as a solvent for the explosive process. Also it is used to produce the methylamine and act as a stabilizer. It has low toxicity and so does not produce any hazardous activities to the environment.