Acetaldehyde Formula
It is an organic chemical compound which is a colourless liquid or gas with highly flammable substance in a liquid state. The systematic IUPAC name is ethanol. During organic synthesis, it act like as a electrophile. Naturally it occurs in the coffee, ripe fruit and grapes. It has consists of pathways of exposure that including air, water, land and groundwater. This is usually contains two carbon atoms with five single bonds and one carbon oxygen double bond.
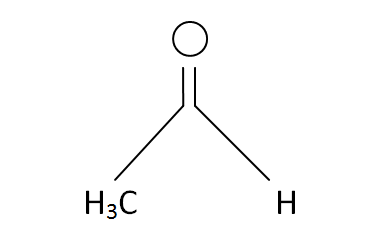
Structural Formula
This is the structural formula of the acetaldehyde:
Chemical Formula
The chemical formula of the acetaldehyde is CH3CHO or C2H4O. In an exothermic reaction the partial oxidation of ethanol is reacted with oxygen and the result is obtained the ethanol. The catalyst is given to this process is 500-650C.
Condensation Reactions
During the condensation reactions the compounds that is Grignard reagents and organolithium is reacted with the methanol and produced hydroxyethyl. To yield imines this acetaldehyde is reacted with amines. These imines are directly used in the aldol condensation. This is known as the building blocks of the heterocyclic compounds.
Physical Properties
The melting point and the boiling point of the acetaldehyde is -123.37C and 20.2C. The maximum density of the ethanol is 788kg/m3. It has contains the molar mass is 44.053g.mol-1. It is catalyzed by the alcohol dehydrogenase. The explosive limit is upto 60%. The molecular structure that contains is called as the trigonal planar.
Chemical Properties
It is similar to the formaldehyde chemical properties. In organic synthesis, it has used as the catalyst of an electrophile. It is used to derive the chemical properties of hydroxyethyl with the reaction of Grignard reactions. It is used to act like the intermediate of pentaerythirtol.
Uses
Acetaldehyde is primarily used as a precursor to acetic acid. It is used to manufacturing such as resin, disinfectants, perfumes and drugs. It is used in the production of polyvinyl acetate and acetic acid.