Potassium Hydroxide
Potassium hydroxide is an inorganic compound that has used for well numerous potassium chemicals. It looks like a white solid and dangerous corrosive. It is basically occurs in base nature. It has a hygroscopic pellets and strong oxidizing agent. Potassium hydroxide contains high stability power. It is the form of series of crystalline hydrates. The systematic IUPAC name is known as potassium hydroxide. The chemical or molecular formula of potassium hydroxide is KOH. It is commonly called as caustic potash.
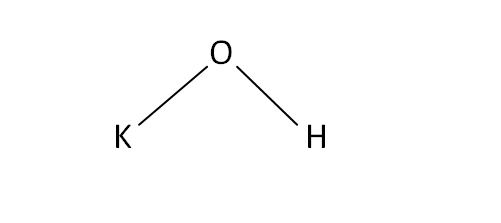
Structural Formula
This is the structural formula of the potassium hydroxide:
Chemical Formula
The chemical formula of the potassium hydroxide is KOH.
Preparation Method
When the potassium chloride is reacted with the water it gives the result of potassium hydroxide. The chloride and hydrogen gas is the byproduct. By the precipitation process it should be remained gas products until its evaporation. Then it separates the anodic and cathodic in the electrolysis cells. They participate in the acid-base equilibrium. It is used to displace halides and other leaving groups.
2KCl + 2H2O → 2KOH + Cl2 + H2
Physical Properties
| Melting point | 360C |
| Boiling point | 1327C |
| Molecular weight | 56.11g/mol |
| Density | 2.12g/cm3 |
| Solubility in water | 162.9g/100mL(100C) |
| Acidity | 14.7 |
| Crystal structure | Rhombohedral |
| Appearance | White solid deliquescent |
| Magnetic susceptibility | -22.0 x 10-6 cm3/mol |
| Solubility | Soluble in alcohol, insoluble in ether and liquid ammonia |
Chemical Properties
The potassium hydroxide is a rhombohedral in structure. It has high volatile point and non inflammable substances. At room temperature it could be the range of orientation group such as 2.6 to 3.15 in the distorted. In the exothermic process it dissolves water quickly.
Uses
It is used to manufacture of soft soaps and liquids detergents. In alkaline batteries it acts as a electrolyte. For example nickel iron batteries is used the potassium hydroxide. In food industry it used to the creating foods thickener. It also used to disinfect surfaces and resist the corrosion materials.