Potassium Bromide
Potassium bromide is a colourless crystal and also typically odourless compounds. It is pungent bitter in taste with saline flavor. In the aqueous solution the pH level of potassium chromate is maintained at 7. It has consists of potassium cations and bromide anions. Moreover it could be lead to the quickly disassociated with the ionic salts and disappears within the minutes. If you consumed at more concentrations it will lead to the irritating the mucous membrances. The systematic IUPAC name is known as potassium bromide . The chemical or molecular formula of potassium bromide is KBr.
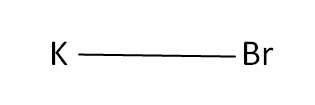
Structural Formula
This is the structural formula of the potassium bromide:
Chemical Formula
The chemical formula of the Potassium bromide is KBr.
Preparation Method
It is prepared by the traditional method by the reaction of potassium carbonate is reacted with the iron bromide and it undergoes to treating with the scrap iron with excess chemical of bromine. The carbon dioxide is given as the byproduct. It can be easily converting the potassium bromide to the potassium nitrate. In the aqeous solution the potassium bromide is reacted with the silver nitrate then it is the formation of potassium nitrate and silver bromide.
4 K2CO3 + Fe3Br8 → 8KBr + Fe3O4 + 4CO2
Physical Properties
| Melting point | 1346C |
| Boiling point | 2615C |
| Molecular weight | 119.002g/mol |
| Density | 2.257g/cm3 |
| Solubility in water | 535g/L |
| Refractive index | 1.559 |
| Crystal structure | Sodium chloride (face centered cubic) |
| Magnetic susceptibility | -49.1×10-6cm3/mol |
| Appearance | White solid |
| Odor | Odourless |
| Solubility | Very slightly soluble in diethyl ether |
Chemical Properties
Potassium bromide has consists of the reversible reactions. In the process of reversible it is converted to the potassium nitrate when it is reacted between the potassium bromide and silver nitrate. The melting and boiling point is very high. It is very slightly soluble in diethyl ether. It looks like a white powder in the appearance.
Uses
Potassium bromide is a halide anticonvulsant. It is widely treatment for veterinary medicine. In the treatment of epilepsy. It is usually used in veterinary such cows,goats, hens etc..,