Magnesium Iodide
Magnesium iodide is a chemical compound which has consists the hydrates and it does not dissolve in the water. By treating with the hydroidic acid it can bes synthesized by using magnesium oxide as the catalyst. It is the combination of the magnesium and iodide. Typically it is the ionic halides and being highly soluble in the water. It can decomposed at the highly moderate temperatures. The systematic IUPAC name is known as magnesium iodide . The chemical or molecular formula of magnesium iodide is MgI2.
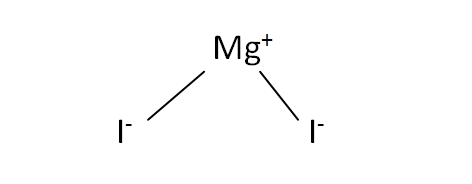
Structural Formula
This is the structural formula of the magnesium iodide:
Chemical Formula
The chemical formula of the magnesium iodide is MgI2.
Preparation Method
There are the three methods to producing the magnesium oxide. They are as follows. When the magnesium oxide is reacted with the hydrogen iodide it gives the magnesium iodide as the product and water as the byproduct. The magnesium hydroxide is reacted with the hydrogen iodide it gives the magnesium iodide is the product and water is the byproduct. Also the magnesium carbonate is reacted with the hydrogen iodide it forms the magnesium oxide as product and carbon dioxide and water as the byproduct.
MgO + 2HI → MgI2 +H2O
Mg(OH)2 + 2HI → MgI2 +2 H2O
MgCO3 + 2 HI → MgI2 + CO2 + H2O
Physical Properties
| Melting point | 637C |
| Boiling point | Decomposes |
| Molecular weight | 278.1139g/mol |
| Density | 4.43g/cm3 |
| Solubility in water | 54.7g/100mL(0C) |
| Refractive index | 1.46 |
| Crystal structure | Hexagonal |
| Magnetic susceptibility | -111×10-6cm3/mol |
| Appearance | White crystalline solid |
| Solubility | Soluble in alcohol, ether and ammonia. |
Chemical Properties
Magnesium iodide is the odourless chemical substances. it is hexagonal in the structure. The melting point is low and it decomposed in the boiling point. It looks like a white crystalline solid in the appearance. It is soluble in alcohol, ether and ammonia.
Uses
It is commonly used in the preparing the chemical compounds for organic synthesis. Medicinally it is diagnosed for many treatments and diseases.