Lithium Hydroxide
Lithium hydroxide is an inorganic compound that has the strong base and a weak alkali metal. It has the composition of lithium and hydroxide. The lithium hydroxide is slightly soluble in the ethanol. When the molecules are dissolved and it releases the form of hydrogen or hydroxide ions. This process is called as the ionization. In the aqueous solution it has ionized 100%. But in the gaseous state it does not reactive and a weakest acid. The systematic IUPAC name is known as lithium hydroxide . The chemical or molecular formula of lithium hydroxide is LiOH.
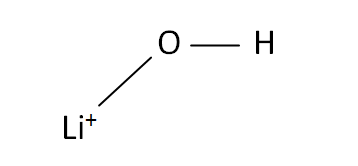
Structural Formula
This is the structural formula of the lithium hydroxide:
Chemical Formula
The chemical formula of the lithium hydroxide is LiOH.
Preparation Method
When the lithium carbonate is reacted with the calcium hydroxide at the heated over 180 degree Celsius, it gives the lithium hydroxide as product and calcium carbonate as the byproduct. It also reacts with the water, hydrochloric acid and sulfuric acid. It gives the various results dependent on the chemical reaction. Lithium hydroxide is more vigorous in the nature.
Li2CO3 + Ca(OH)2 → 2 LiOH + CaCO3
Physical Properties
| Melting point | 462C |
| Boiling point | 924C |
| Molecular weight | 23.95g/mol |
| Density | 1.46g/cm3 |
| Solubility in water | 12.7g/100mL(0C) |
| Refractive index | 1.464 |
| Magnetic susceptibility | -12.3×10-6cm3/mol |
| Appearance | White solid |
| Acidity | 14.4 |
Chemical Properties
Lithium hydroxide is a strong base chemical compound. It is a non inflammable substances. The Refractive index of anhydrous state is greater than the monohydrate state. The melting point is low and the boiling point is high. It has low density and low molar mass.
Uses
Lithium hydroxide is used to absorb unwanted gas. It is manufacturing the lithium salts, soaps and lithium based greases. It is also used for other lithium chemicals such as lithium chloride, lithium bromide and lithium iodide. It protects the radiation from the free electrons.