Zinc Sulfate
Zinc sulfate is an inorganic compound and it has been also non combustible substances. These zinc sulfide prevents the high risk factors. One of the most common forms is crystallization and gives the hepahydrate. It should be colourless compounds. It is very important for the dietary supplement to the human things. The zinc has the positively charged and the sulfate has the negatively charged. The systematic IUPAC name is known as zinc sulfate. The chemical or molecular formula of zinc sulfate is ZnSO4 . It is also known as zincate or white vitriol.
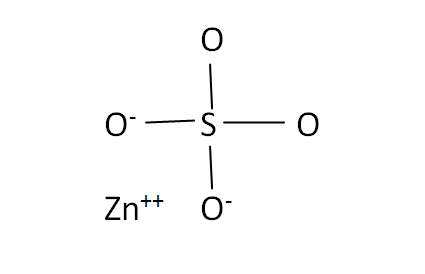
Structural Formula
This is the structural formula of the zinc sulfate:
Chemical Formula
The chemical formula of the zinc sulfate is ZnSO4. It has comprises one zinc atom and four sulfate ions.
What Method the Zinc Sulfate Can be Produced
The zinc sulfate is prepared by the reacting zinc with the aqueous sulphuric acid and it forms the product and hydrogen gas is evolved as the byproduct. It should be high purity chemical compounds. The zinc has the positive ions while the sulfate has the negative ions. The following chemical reactions is given below.
Zn + H2SO4 + 7H2O → ZnSO4.7H2O + H2
Physical Properties
| Melting point | 680C |
| Boiling point | 740C |
| Molecular weight | 161.47g/mol |
| Density | 3.54g/cm3 |
| Solubility in water | 57.7g/100mL |
| Refractive index | 1.658 |
| Magnetic susceptibility | -45×10-6cm3/mol |
| Appearance | White powder |
| Odor | Odourless |
| Solubility | alcohols |
Chemical Properties
Zinc sulfate is a non inflammable substances. it is hazardous while making the products in the industry. It has no taste and appears as white in colour. There is normal melting and boiling point.
Uses
Zinc sulfate is medically used for rehydration therapy. The rayon is produced by using the zinc sulfate. For adult persons it helps for eye drops and lotions. It is used for mordant dyeing. It is widely used for preservative of leathers.