Strontium Nitrate
Strontium nitrate is an inorganic compound that has combination of the following elemens such as nitrogen, oxygen and strontium. The strontium acts as the cathode and the nitrate acts as the anode. It lacks the carbon-hydrogen bonds so we called as the inorganic compounds. Theses sodium nitrate is making the explosive bombs and cracking fireworks which is known as the pyrotechnics process. The systematic IUPAC name is known as strontium nitrate. The chemical or molecular formula of strontium nitrate is Sr(NO3)2.
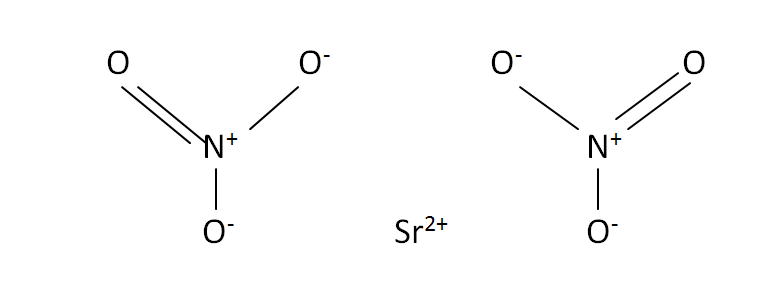
Structural Formula
This is the structural formula of the strontium nitrate:
Chemical Formula
The chemical formula of the strontium nitrate is Sr(NO3)2. It has one strontium and two nitrate compounds.
How it Can be Prepared
The preparation of the strontium nitrate is a well chemical reaction between the nitric acid and strontium carbonate. It gives the water and carbon dioxide as the byproduct. In the laboratory at the vessel beaker it appears as the white crystalline solid. It decomposed at the tetrahydrate stage. The chemical reaction is given as follows.
2 HNO3 + SrCO3 → Sr(NO3)2 + H2O + CO2
Physical Properties
| Melting point | 570C |
| Boiling point | 645C |
| Molecular weight | 211.630g/mol |
| Density | 2.986g/cm3 |
| Solubility in water | 660g/100mL(20C) |
| Refractive index | 1.54 |
| Crystal structure | Cubic |
| Magnetic susceptibility | -57.2×10-6cm3/mol |
| Appearance | White crystalline solid |
| Solubility | Soluble in ammonia. Insoluble in nitric acid. |
Chemical Properties
Strontium nitrate has a mousy odour and it is in the form of white crystalline hygroscopic solid. The refractive index is less than the limit of threshold. It is insoluble in nitric acide. Both the melting and boiling point is low. It has bitter in taste.
Uses
Strontium nitrate is used in red colour flame in fireworks and road flares. It is the oxidizing agent. It can cured the lessening skin irritations. It makes the salts like the other chemical compounds.