Sodium Thiosulfate
Sodium thiosulfate is an inorganic compound that has consists of sodium as cations and sulfate as anions. It is a white or colourless and dissolves well in the water. it interacts with the single bonded atoms which is called as the electronic configuration. These electrons are replaced or exchanged the atoms from their substances. sodium thiosulfate also neutralizes the colour removing effects and increased the level of pH in the liquid solutions. The systematic IUPAC name is known as sodium thiosulfate . The chemical or molecular formula of sodium thiosulfate is Na2S2O3. The other names of sodium thiosulfate is sodium hyposulfite or hyposulfite of soda.
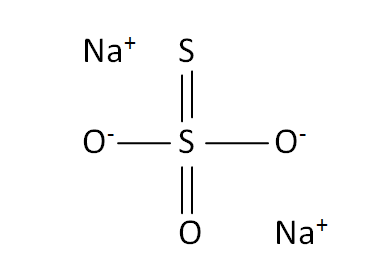
Structural Formula
This is the structural formula of the sodium thiosulfate:
Chemical Formula
The chemical formula of the sodium thiosulfate is Na2S2O3.
Preparation method
When the sodium hydroxide is reacted with the sulfur at the given boiling point it produced the sodium thiosulfate. Sodium sulfide and water gives as an byproduct. In the laboratory process, it has produced the sodium thiosulfate by heating the solutios in the aqueous form. When it is heated at the temperature of 300C it decomposed or converted to sodium sulfate and sodium polysulfide.
6 NaOH + 4S → Na2s2O3 + 2Na2S + 3H2O
Physical Properties
| Melting point | 48.3C |
| Boiling point | 100C |
| Molecular weight | 158.11g/mol |
| Density | 1.667g/cm3 |
| Solubility in water | Soluble |
| Refractive index | 1.489 |
| Crystal structure | Monoclinic |
| Appearance | White crystals |
| Odor | Odourless |
| Solubility | Negligible in alcohol |
Chemical Properties
Sodium thiosulfate is a non inflammable substances and also more hazardous to the environment. The melting and boiling point is very low. It is monoclinic in structure. The range of the refractive index is less than 10. It looks like a white crystals in the appearance. It is soluble in water and bitter in taste.
Uses
Sodium sulfate is used in the treatment of cyanide poisoning agent. It is medically cured for dermatophytosis. It is very important for analytical chemistry. Sodium thiosulfate salts used for photographic fixers.