Potassium Chlorate
Potassium chlorate is an inorganic compound which was discovered by the French chemist claude Louis berthollet. It contains the potassium, oxygen, chlorine as in the ratio of 1:3:1. Potassium chlorate is in its pure form when it kept at room temperature. It has contains the strong oxidizing agent which is used for fireworks in the industries. The systematic IUPAC name is known as potassium chlorate . The chemical or molecular formula of potassium chlorate is KClO3. It is also known as berthollet salt.
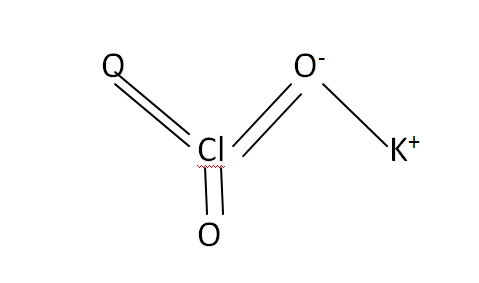
Structural Formula
This is the structural formula of the potassium chlorate:
Chemical Formula
The chemical formula of the Potassium chlorate is KClO3.
Preparation Method
The potassium chlorate is prepared by the three ways. They are electrolysis method, disproportionation method and caustic potash. But it is mainly prepared in the liebig process. In this process the chlorine is passed and heated with the calcium hydroxide. Further then adding the potassium chloride in it to form the potassium chlorate. The chemical reactions is given as follows.
6Ca(OH)2 + 6Cl2 → Ca(ClO3)2 + 5CaCl2 + 6H2O
Ca(ClO3)2 + 2KCl → 2KClO3 + CaCl2
Physical Properties
| Melting point | 356C |
| Boiling point | 400C |
| Molecular weight | 122.55g/mol |
| Density | 2.34g/cm3 |
| Solubility in water | 3.13g/100mL |
| Refractive index | 1.40835 |
| Crystal structure | Monoclinic |
| Magnetic susceptibility | -42.8×10-6cm3/mol |
| Appearance | White crystals or powder |
| Solubility | Soluble in glycerol |
Chemical Properties
Potassium chlorate is a harmful substances and more hazards to the environment. The melting and boiling point is very low. It has a low density and moderate molar mass. It is monoclinic in structure. It is insoluble in glycerol.
Uses
Potassium chlorate is used for generating oxygen gas in college and schools. It is manufacturing the pesticides for agriculture fields. It is widely used for manufacturing of paper. By using potassium chlorate the match box was made. It is produced the firearms and percussion caps.