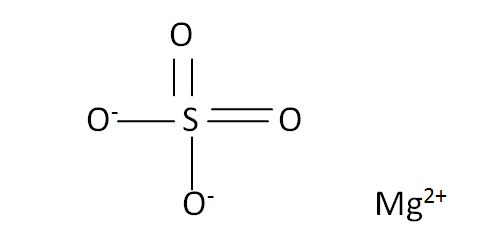Magnesium Sulfate
Magnesium sulfate is a chemical compound that has the combination of the magnesium and sulfate. It is commonly the magnesium as the cation and sulfate as the anion. It is essential for the nutrients. In the anhydrous form it has consists for the several forms of minerals and is significant to the water. it is soluble in the water and not in the ethanol. The systematic IUPAC name is known as magnesium sulfate. The chemical or molecular formula of magnesium sulfate is MgSO4.
Structural Formula
This is the structural formula of the magnesium sulfate:
Chemical Formula
The chemical formula of the magnesium sulfate is MgSO4.
Preparation Method
The magnesium sulfate can be prepared by the reaction between the magnesium carbonate with the sulphuric acid. It is commonly obtained from the natural sources such as lake beds etc. These chemical compounds is used for the manufacturing various agricultural products. It cures the many diseases. It crystallizes the heptahydrate The chemical reaction is given as follows.
MgCO3 + H2SO4 → MgSO4 +H2CO3
Physical Properties
| Melting point | 1124C |
| Boiling point | Decomposes |
| Molecular weight | 120.366g/mol |
| Density | 2.66g/cm3 |
| Solubility in water | 26.9g/100mL(0C) |
| Refractive index | 1.523 |
| Crystal structure | Monoclinic |
| Magnetic susceptibility | -50×10-6cm3/mol |
| Appearance | White crystalline solid |
| Solubility | Slightly soluble in alcohol, glycerol. Insoluble in acetone. |
| Odor | odourless |
Chemical Properties
Magnesium sulfate is a white crystalline solid and odourless chemical compounds. It is insoluble in the acetone and it is soluble in alcohol and glycerol. It is monoclinic in structure. The magnetic susceptibility is very low. It has low density and medium molecular weight. It decomposed at the boiling point.
Uses
In medical the magnesium sulfate is used for the treatment of asthma and eclampsia. In agriculture it increase the magnesium and sulfur content in the soil. In food preparation it is used to brewing the salt to manufacturing the beer. The cement is manufactured by using in the construction buildings.