Barium Fluoride
Barium fluoride is an inorganic compound and naturally it occurs in the frankdickstonite which is called as the rare mineral. When it manages the fluorite structure and then it adopts the pbcl2 due to the high pressure. During the transmission of the UV range it degrades all the exposures in the environments. It is highly resistant when it is compared to the other fluorides. Barium fluoride is very quick hard substance and it can be quite easily away from the fracturing.The systematic IUPAC name is known as Barium fluoride. The chemical or molecular formula of Barium fluoride is BaF2.
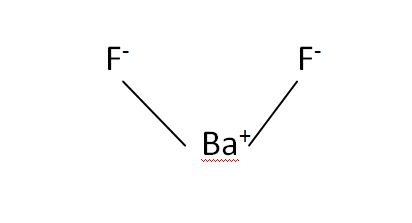
Structural Formula
This is the structural formula of the Barium fluoride:
Chemical Formula
The chemical formula of the Barium fluoride is BaF2.
Optical Properties
The barium fluoride is one of the fastest scintillators for the detection of gamma rays, x-rays and other high energy radiation. It does not emitting the ultraviolet rays. The refractive index is about 1.46. by using the pulse shape discrimination techniques the gamma photons are separated simultaneously.
Physical Properties
| Melting point | 1368C |
| Boiling point | 2260C |
| Molecular weight | 175.34g/mol |
| Density | 4.89g/cm3 |
| Solubility in water | Slightly soluble |
| Refractive index | 1.557 |
| Crystal structure | Fluorite(cubic) |
| Magnetic susceptibility | -51.1×10-6cm3/mol |
| Appearance | White cubic crystals |
Chemical Properties
Barium fluoride is a white cubic crystals. It is slightly soluble in water. the melting and boiling point is very high. The refractive index is greater than 1. It is fluorite cubic in structure. It has low density and molecular weight. It is non-inflammable substance and highly toxic chemical compounds.
Uses
Barium fluoride is used to make opticals such as lens. It is also used in the manufacture of carbon brushes. It is mainly used glass making. It is used for the detection of high energy neutrons.