Potassium Bicarbonate
Potassium bicarbonate is an inorganic compound that is used to manufacturing the soap and glass. It is called as the pearl ash and carbonate potash. It is a hygroscopic and odourless and same as the alkaline. It is insoluble in acetone and readily soluble in water. The systematic IUPAC name is known as potassium hydrogencarbonate. The chemical or molecular formula of potassium bicarbonate is KHCO3 . The other names is potassium acid carbonate.
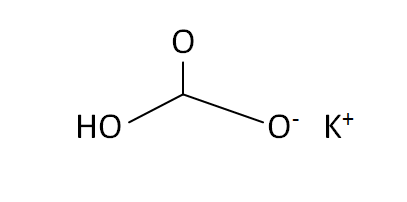
Structural Formula
This is the structural formula of the potassium bicarbonate:
Chemical Formula
The chemical formula of the potassium bicarbonate is KHCO3.
Preparation Method
When the potassium carbonate is reacted with the carbon dioxide and water, it gives the results of potassium bicarbonate.
K2C03 + CO2 + H20 → 2 KHCO3
It can be substitute for baking soda and ingredient low sodium baking powders. It should be an expensive and nontoxic base. it has decomposed at the high level temperatures such as 100 and 120C.
2KHCO3 → K2C03 + CO2 + H2
Physical Properties
| Melting point | 292C |
| Boiling point | 881.4C |
| Molecular weight | 105.115g/mol |
| Density | 2.17g/cm3 |
| Solubility in water | 22.4g/100mL(20C) |
| Solubility | Practically insoluble in alcohol |
| Acidity | 10.329 |
| Appearance | White crystals |
Chemical Properties
It is mainly used in the reagent materials and especially in the condensed aerosol fire suppression. It regulates the ph level of the toxic gases. It is a non inflammable substances. The effect of effervescence should be soften the chemical compounds and increased to the taste of baking soda.
Uses
Potassium bicarbonate is a leavening agent in baking processes to liberate the carbon-dioxide. It is a dry chemical used in fire extinguishers. It has a several applications in ph regulation. It is mainly used in agricultural field to neutralize the acidic solids. It is also used in wine making. It is also a buffering agent to control the Ph of several medications.