Aluminium Chloride Formula
Aluminium chloride has consists of aluminium and chlorine atoms that are in the ration of 1:3 to dissolve in the six waters of hydration. It is mainly produced from the aluminium metal and often it is called as the lewis acid. It is also known as aluminium trichloride. Whenever the reversible changes occur it converts from polymer to a monomer. The systematic IUPAC name is known as aluminium chloride. The chemical or molecular formula of aluminium chloride is AlCl3.
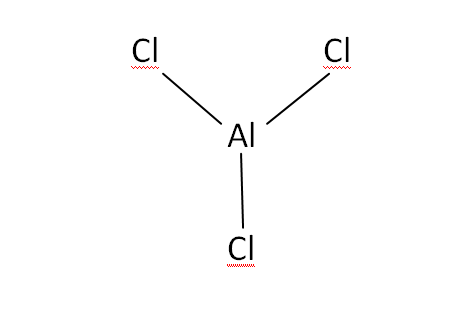
Structural Formula
This is the structural formula of the aluminium chloride:
Chemical Formula
The chemical formula of the aluminium chloride is AlCl3.
Types of Structure
Anhydrous
It has a sheet like layered with cubic closed package chloride ions. It has low density at the liquid phase. When it is in melted state it has produced the dimer Al2Cl6 reacts with tetracoordinate aluminium. This is also found in the vapour phase.
Hexahydrate
The hexahydrate has a six side molecules that has consists of the cations and anions. It looks like a solid state crystal structure. The octahedral molecular geometry has been produced in the hydrated form of aluminium chloride. It has a coordinatively saturated in friedal crafts alkylation.
Physical Properties
| Melting point | 180C |
| Boiling point | 192.6C |
| Molecular weight | 133.341 g/mol |
| Density | 2.48 g/cm3 |
| Solubility in water | 490 g/l(100C) |
| Vapour pressure | 133.3 pa |
| Appearance | White or pale yellow solid |
| Viscosity | 0.35cP(197C) |
| Crystal structure | monoclinic |
Chemical Properties
The aluminium chloride has mixed with the liquid water molecules and are displaced to the H2O molecules to form the hexahydrate[Al(H2O)6]Cl3. The metal aquo complexes has the ionization of the aquo ligands.
Al(H2O)6Cl3 Al(OH)3+3HCl+3H2O
Uses
Aluminium chloride is used to manufacturing for ethylbenzene and anthraquinone. It is often displaced by the zeolites. It contains the groups called the aldehyde. By fischer-hafner synthesis the reaction of benzene and phosphorus trichloride can be produced the dichloro phenyl phosphine. It is used to making the induce variety of hydrocarbon couplings and rearrangements.